Abstract
Background
Children with Sickle Cell Anemia (SCA) are at high risk for overt or silent strokes, and this risk may be mitigated by hematopoietic stem cell transplant (HSCT). Our research group has shown that when cerebral oxygen delivery is compromised due to anemia in SCA, the cerebral blood flow (CBF) and brain oxygen extraction fraction (OEF) increase to maintain brain tissue oxygen delivery. We used novel magnetic resonance imaging (MRI) sequences to measure OEF and CBF in children with SCA treated with HSCT, testing the hypothesis that after HSCT, OEF and CBF will be similar to control values.
Methods
Children with SCA were enrolled in a pilot prospective, observational study of MRI-measured OEF and CBF. Brain MRI exams were performed without sedation on a 3T Siemens scanner. The MRI protocol included fluid-attenuated inverse recovery (FLAIR) sequences in two planes to evaluate for strokes, asymmetric spin echo to measure tissue-level OEF, and pseudo-continuous arterial spin labeling perfusion-weighted imaging to measure CBF. HSCT recipients underwent paired brain MRI 1-2 months before and >1 year after HSCT. Controls, non-anemic siblings of SCA patients, had a single brain MRI. Pre- and post-HSCT CBF and OEF were compared within subjects and between the HSCT and control cohorts using non-parametric tests. Bivariate correlations between OEF or CBF and hemoglobin (Hb) or Hb S concentrations were tested with Spearman's rho. The level of significance was specified as p < 0.05 after adjustment by the Benjamini-Hochberg procedure.
Results
Four children (two male, median age 10 years, range 8-21 years) underwent HSCT: one had prior overt stroke, one had silent strokes, and two had no neuroimaging abnormalities. All received a reduced intensity preparative regimen of alemtuzumab, melphalan, and fludarabine. One subject received marrow from an HLA-matched unrelated donor without Hb S trait; two received marrow from HLA-identical siblings. One child who had rejected a HSCT from an HLA-identical sibling underwent re-conditioning with antithymocyte globulin and cyclophosphamide and received CD-34-selected peripheral stem cells from the same donor. All sibling donors had Hb S trait. Brain MRIs of 20 non-anemic sibling controls of median age 11 years (range 6-17 years) were included for comparison.
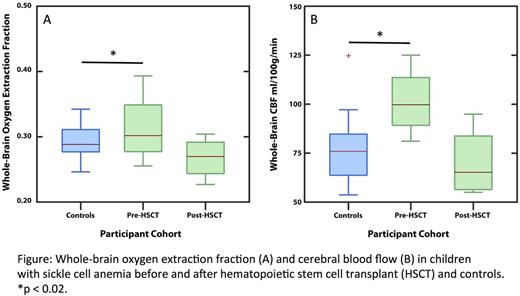
Median OEF and CBF were significantly elevated pre-HSCT compared to controls (OEF median 0.355 vs 0.288, p = 0.012; CBF median 99.8 vs 65.1 ml/100 g/min, p = 0.018; Figure). Median post-HSCT OEF and CBF were lower than pre-HSCT values (OEF median 0.270 vs 0.355, CBF median 65.1 vs 99.8 ml/100 g/min, p = 0.13 for both), but these analyses had low power to detect significant differences due to the small sample size. Post-HSCT, recipients' OEF and CBF were similar to controls (OEF median 0.270 vs 0.288, p=0.18; CBF median CBF 65.1 vs 75.8 ml/100 g/min, p = 0.42). None of the HSCT recipients had new overt or silent strokes on FLAIR imaging or developed severe acute or chronic GVHD after HSCT.
Pre-HSCT, recipients were anemic compared to controls (median Hb 9.8 vs 12.8 g/dl, p = 0.018). Pre-HSCT Hb S concentrations were lower than expected for SCA due to recent red blood cell transfusions (median 50.6%, range 39.7-77.6%). At the post-HSCT imaging timepoint, recipients' anemia had resolved (median Hb 12.4 g/dl, range 11.3-15.7 g/dl), and Hb S concentrations were consistent with their donors' levels. When all pre- and post-HSCT measurements were compared, OEF had a strong negative correlation with Hb (Spearman's rho = -0.905, p = 0.002) and a strong positive correlation with Hb S concentration (Spearman's rho = 0.970, p < 0.001). CBF had a weaker negative correlation with Hb (Spearman's rho = -0.714, p = 0.047) and positive correlation with Hb S concentration (Spearman's rho = 0.731, p = 0.040).
Conclusion
In this cohort, children with SCA had normalization of OEF and CBF after successful HSCT. OEF elevation was strongly correlated with Hb S concentration and inversely correlated with Hb concentration in these HSCT recipients. Our data suggest that when curative HSCT corrects hemolytic anemia, OEF and CBF normalize. This physiologic change may provide protection against strokes following HSCT for SCA.
Hulbert: Pfizer: Other: Spouse employment at Pfizer. Fields: Proclara Biosciences: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal